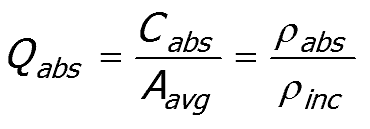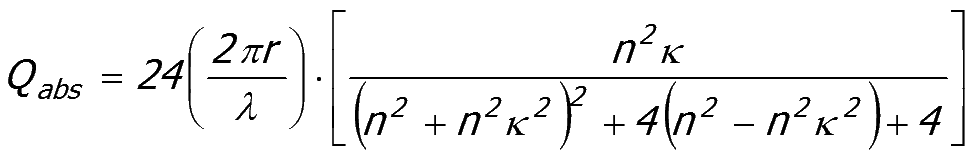/* ========== Appearance ========== */
|
H52: Pigmented Materials
Light Absorption and Particle Size
|
© James H Nobbs
[Colour4Free]
|
Introduction
In
the two sections H52 and H53, the relationships between the optical
properties of a pigmented material and the optical properties of the
individual pigment particles are explored.
In Section H52, the following subjects are considered:
- The absorption properties of a single particle and the concept of "absorption efficiency" is introduced.
- The relationships between the properties of the single particles and the properties of a pigmented material.
|
The colour of a pigmented coating material depends on the types and amounts of pigments present in the material.
The
colour also depends on the size of the pigment particles and how well
the pigment particles are dispersed within the coating.
This
relationship is illustrated by the result of a simple test of the state
of dispersion, the "finger rub test", one of the QC tests described in
section H26, "QC Tests for Colorants and Inks".
Example of a "Finger-Rub Test"
The
test panel from a finger rub test of a paint that has a poor dispersion
of the pigment particles is shown in Figure 1. In this paint,
some of the pigment particles are present in loosely bound clusters,
they are flocculates.
A
few minutes after the application of the air-drying pigmented coating,
the surface of the coated panel is gently rubbed by a finger, using a
circular movement. The poorly dispersed clusters breakdown during
the rubbing, this increases the level of dispersion in the rubbed areas
by the break down of clusters into individual particles.
|
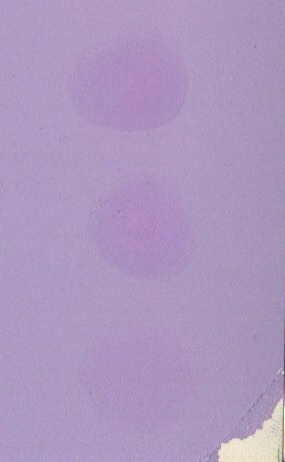
Figure 1: Finger rub test of the state of dispersion.
|
This
results in an increase in the depth of colour in the rubbed region, as
illustrated by the rubbed patches in the central region of Figure 1.
Light absorbed by a single particle
|
Each
particle in a pigmented material may interact with the incident
light. The interaction that occurs for an individual particle
will depend on the intensity of the light and the surface area that the
particle presents to the beam.
For
particles of an irregular shape, the presented area depends on the
orientation of the particle relative to the direction of the beam, as
illustrated in Figure 2.
|
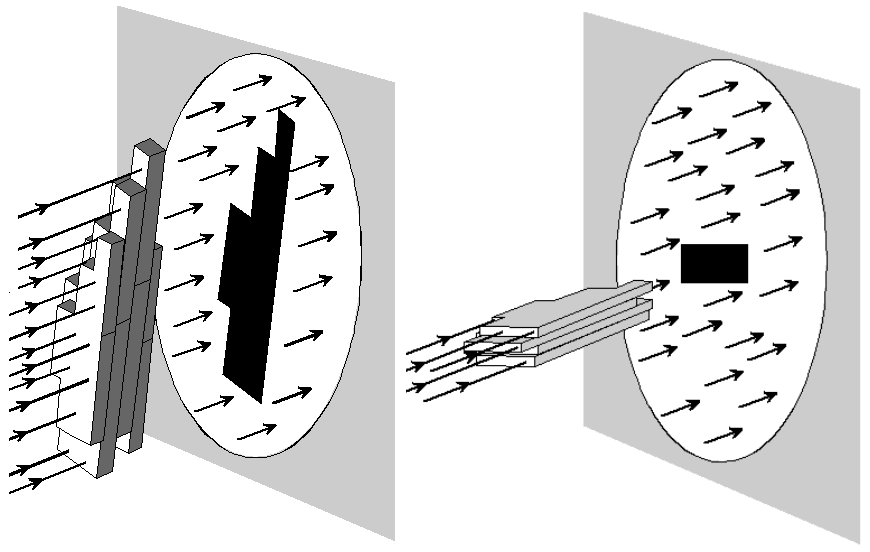
|
|
Figure 2: Cross sectional area and orientation. |
It is usual to assume that the particles in a dispersion are randomly oriented and an average cross sectional area, Aavg, is used to represent the area that a particle presents to a light beam.
Power incident on a single particle ρinc
The power incident on a single particle ρinc , averaged over all orientations, is determined from the
|
incident intensity (Iinc) multiplied by the area presented to the beam (Aavg)
|
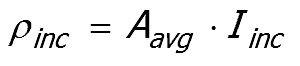
|
Power absorbed by the particle ρabs
The power absorbed by the particle ρabs ,
will depend on the optical properties of the particle. If the
material is colourless and transparent then no light is absorbed.
If the particle material is strongly absorbing, carbon-black for
example, then almost all of the light may be absorbed.
The
variation in absorption is characterised by defining an effective
cross-sectional area, known as the absorption cross-section area Cabs .
|
It follows that the power absorbed by the particle is given by
|
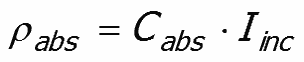
|
The ratio of geometric area (Aavg) to the effective cross-section Cabs, describes the efficiency of the pigment particle at absorbing the incident light.
|
The absorption efficiency Qabs of the pigment particles is defined as
|
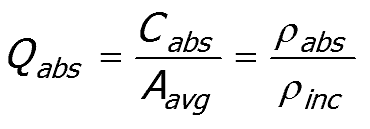
|
|
Rearranging the equation provides the power absorbed by the particle
|
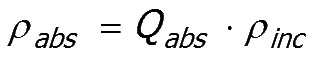
|
|
Substituting Aavg Iinc for ρinc gives;
|
Equation 52:1
|
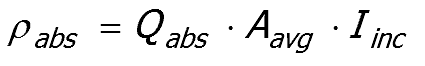
|
The
absorption efficiency has no dimensions; it is the ratio of the
effective cross-sectional area of the particle to the geometrical
cross-sectional area.
The value of the absorption efficiency depends on;
|
The size of the particle
|
The refractive indices of the particle
|
|
The wavelength of the light
|
The refractive indices of the surrounding material
|
|
The shape of the particle
|
|
Dependence of absorption efficiency on particle size
Fortunately, the way in which the absorption efficiency of a pigment particle changes with particle size is simple.
- The absorption efficiency starts at zero for zero size.
- For small particles compared to the wavelength of light, the efficiency increases linearly with the size.
- Beyond a certain size, which is dependent on the particle properties, the rate of increase starts to fall.
- Eventually
the efficiency reaches an upper limit value of 1 or 100%. It then
remains constant for further increase in the size.
Typical efficiency versus size relationships are shown in Figure 3 for three different types of pigment.
The
efficiency is determined for spherical particles that are surrounded by
a colourless, transparent medium with properties that are typical for a
paint medium and for a plastic.
The optical properties of the materials shown in Figure 3 are characterised by their refractive index (n ) and attenuation coefficient (κ ).
|
Material
|
Optical properties
|
|
Inorganic pigment
|
n1 = 3.00, κ = 0.05
|
|
Organic pigment
|
n1 = 1.60, κ = 0.50
|
|
Carbon black pigment
|
n1 = 1.60, κ = 1.00
|
|
Medium
|
n0 = 1.50, κ = 0.00
|
|
Small sized spheres
Some
of the light that is incident on the surface is not reflected and
will be transmitted into the sphere. For small spheres, the short
distance travelled by the light within the sphere means that only part
of the light is absorbed, the rest is transmitted out of the sphere and
the absorption efficiency of the particle is much less than one.
Medium sized spheres
As
the sphere becomes larger, more of the light passing into the sphere is
absorbed because of the greater distance it travels. The
absorption efficiency of the particle increases as the size increases.
|
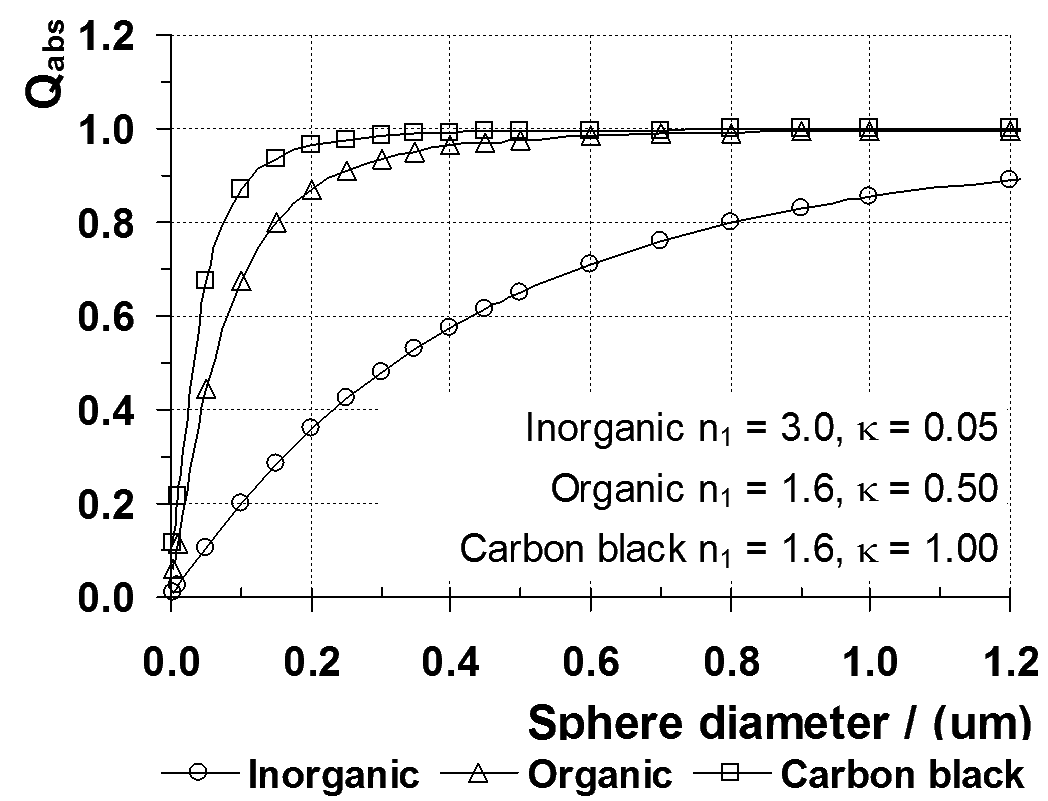
Figure 3: Absorption efficiency for different types of pigment
|
Large sized spheres
The
distance travelled by the light within the sphere is so great that none
of the light passing into the sphere is transmitted through it.
The absorption efficiency has reached a constant, limiting value.
Large particle limit
Figure 3 shows that Qabs trends
towards a value of 1.0. However, this is not the correct limiting
value for a large particle because only the fraction of the light that
is not scattered by the particle can be absorbed.
For
a large particle, scattering is by boundary reflection and the upper
limit to the absorption efficiency is 1 minus the boundary reflection
at the medium to particle interface.
|
The correct large particle limit is
|
Equation 52:2;
|
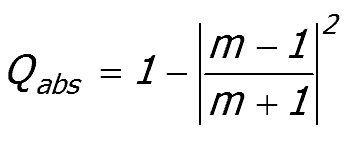
|
Where m = (n1/n0)
Small particles (less than ~0.4 um)
Rayleigh's law of absorption
For
very small particles, smaller than the wavelength of light, Rayleigh
determined an equation for the absorption efficiency of small spheres
of radius r.
|
|
Equation 52:3;
|
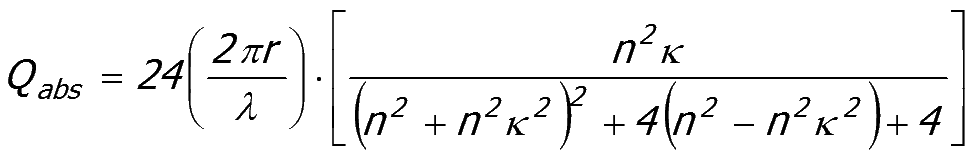
|
Rayleigh's absorption law has several consequences:
- The
absorption efficiency of small particles is linearly dependent on their
size. The efficiency increases with increase in particle size.
- For the same value of the attenuation coefficient κ, short wavelengths are absorbed more strongly than long wavelengths.
- The
absorption efficiency is only weakly dependent on n, the real part of
the refractive index ratio. This is not obvious from the form of Equation 52:3.
The Rayleigh equation applies to small particles only, particles with dimensions that are smaller than the wavelength of light.
Medium to large size of particles (0.3 um to 6um)
Mie theory of absorption
The
"anomalous diffraction" approximation to the Mie theory is used to
estimate the absorption efficiency for the sizes of particle similar to
the wavelength of light and larger. As shown earlier, Qabs approaches a constant value for large particles, large compared to the wavelength of light.
The Mie equations reproduce the correct general shape for a plot of Qabs against the size parameter but the magnitude of the Qabs values are systematically in error.
|
|
Equation 52:4;
|
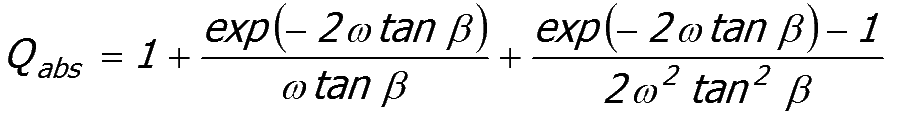
|
|
Where
|
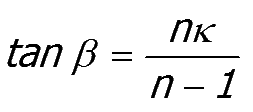
|
|
|
and ω is a dimensionless size parameter
|
Equation 52:5;
|
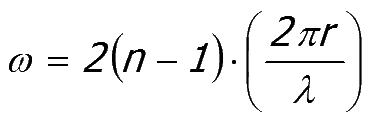
|
Conclusions, single particles
It
is possible to relate the optical properties of a particle of a
material to the molecular properties by means of the refractive index n and the attenuation coefficient κ.
A study of the absorption efficiency of particles has shown that
- The absorption efficiency of particles much smaller than the wavelength of light is linearly proportional to their size.
- Above a certain size, the absorption efficiency of a particle is independent of the size of the particle.
- The absorption efficiency of particles depends on the attenuation coefficient of the material.
Absorption coefficient of a pigmented material
The primary definition of an absorption coefficient of a material is the parameter (ε )
within the Lambert-Beer-Bougeur law. This law principally applies
to light travelling within a coloured material that absorbs, but does
not scatter light.
Lambert-Beer-Bougeur law absorption coefficient (ε )
Imagine a beam of collimated light ( Iinc ) passing through a thin slice within a material that does not scatter light.
The law proposes that the of the intensity lost by absorption ( dIinc ) is proportional to the product of the intensity ( Iinc ), the absorption coefficient ε and the thickness of the slice ( dx ).
|
Intensity lost by absorption within the slice.
|
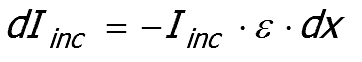
|
The volume element ( dv ) involved in the absorption process, remembering that intensity has units
|
of power per unit area, is given by
|
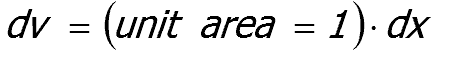
|
|
It follows that the number of particles in the slice is
|
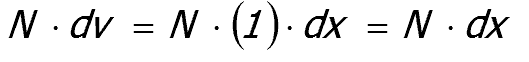
|
where N is the number of particles per unit volume of material.
|
From this we can obtain the power absorbed per particle.
|
Equation 52:6;
|

|
|
Equation 52:1 shows that the absorption power of a single particle is
|
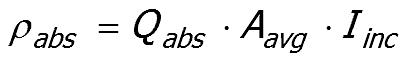
|
Comparison of these two equations provides the absorption coefficient of a pigment dispersion (ε ),
|
in terms of the properties of the individual particles.
|
Equation 52:7;
|
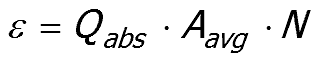
|
Dependence of ε on particle size
It
is well known that the tinting strength within the material of some
types of pigment can increase dramatically during the milling and
dispersion process. The absorption coefficient for a dispersion
containing a fixed volume fraction V of mono-sized spheres is related to the particle size
|
by the equation
|
Equation 52:8;
|
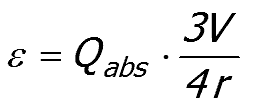
|
A dimensionless size parameter (X = 2πr/λ) can be used to separate the RHS of Equation 52:8 into a size dependent term (Qabs/X ) and a volume fraction dependent term (3πV/2λ).
|
|
Equation 52:9;
|
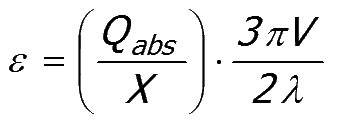
|
Figure 4 shows the prediction of the way the absorption coefficient (ε)
of a pigmented material depends on the diameter of the spherical
pigment particles. The material contains a fixed volume fraction
of pigment, so that the value of (3πV/2λ) is constant.
is a plot of (Qabs/X )
against particle size for a dispersion of three different types of
spherical particles immersed in a medium of refractive index n0 = 1.50.
The plots shown in Figure 4 are typical for:
|
Material
|
Optical properties
|
|
Inorganic pigment
|
n1 = 3.00, κ = 0.05
|
|
Organic pigment
|
n1 = 1.60, κ = 0.50
|
|
Carbon black pigment
|
n1 = 1.60, κ = 1.00
|
|
Medium
|
n0 = 1.50, κ = 0.00
|
|
Figure
4 shows that, in the limit of very small particle size, the absorption
coefficient of the dispersion has a constant upper value.
For
dispersions of particles of strongly absorbing materials such as
organic pigments and carbon black, the coefficient remains constant
with increase in size until, above a certain size, the absorption
coefficient decreases with increase in particle size.
The rapid decrease for can be explained by noting that for these types of pigment, Qabs rapidly approaches a constant value of nearly 1 as size increases.
|
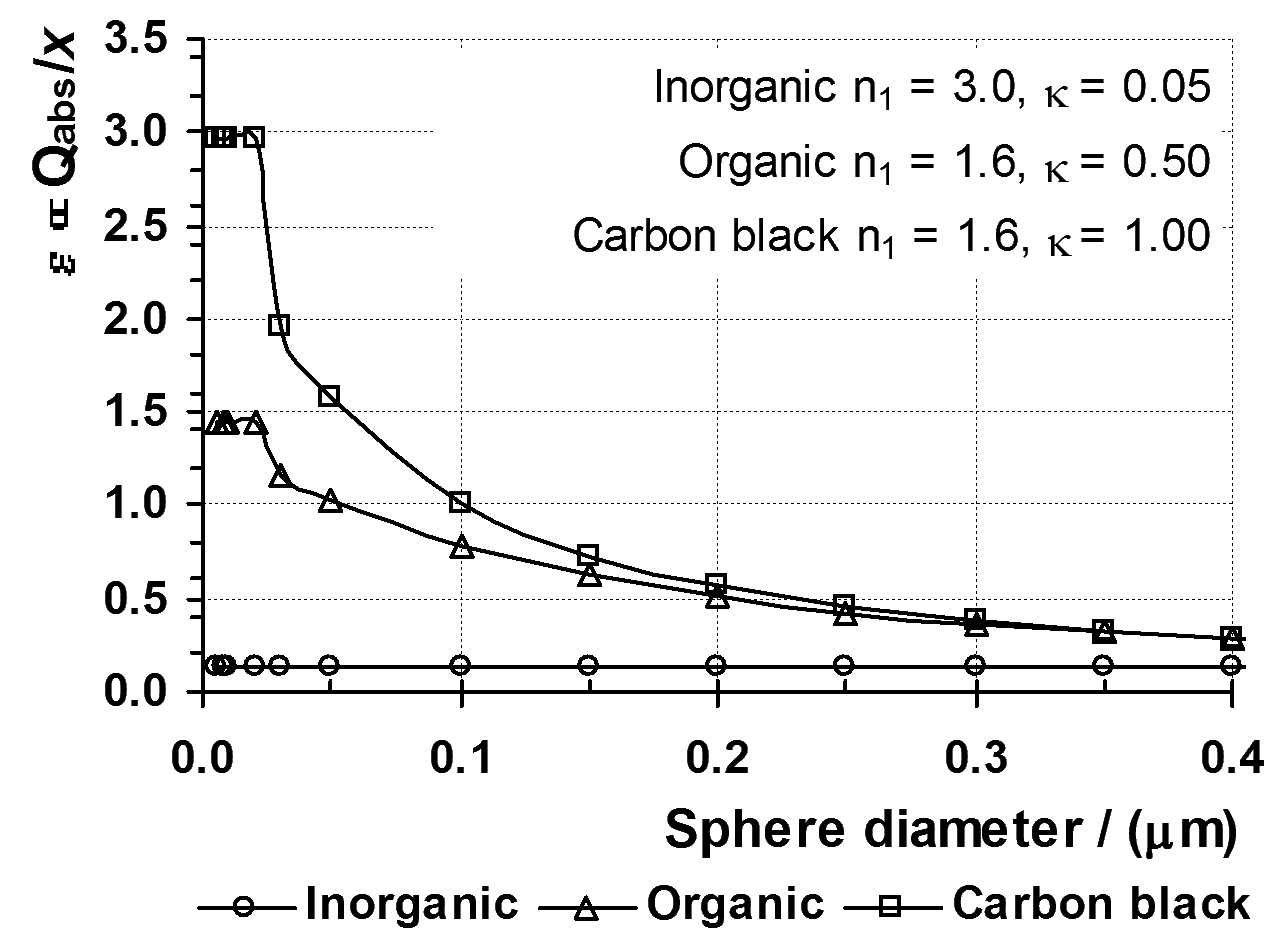
Figure 4: Absorption coefficient for dispersions of 3 types of pigment.
|
It follows from Equation 52:9, that when Qabs is constant the absorption coefficient (ε) of the material will decrease as the particle size (r) increases in proportion to (1/X ) = (λ/2πr ).
|
According
to Figure 4, the absorption coefficient of the dispersion of inorganic
pigment does not change with particle size. This is a false
impression created by the limited range of sizes shown in Figure 4.
Figure
5 is a plot of the absorption coefficient values of the dispersion of
inorganic pigment over a wider range of sizes. The figure shows that
the overall pattern of the size dependence for inorganic pigments is
the same as that of organic pigments and carbon black.
|
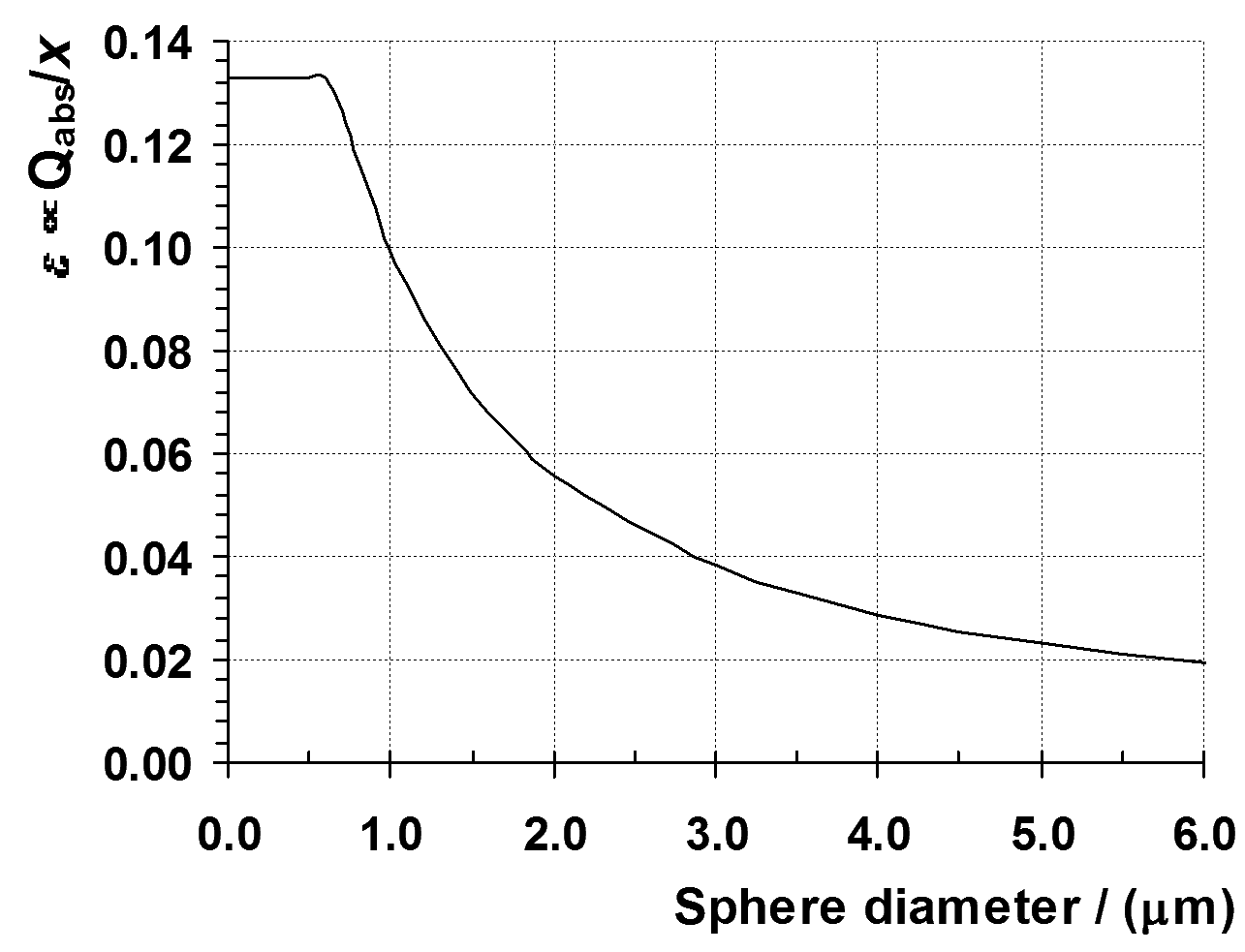
Figure 5: Absorption coefficient for a dispersion of inorganic pigment
|
|
It
is the absorption versus size relationship shown in Figures 4 and 5
that is apparent in the colour depth increase seen for a "finger rub
test" panel when the test is applied to a poorly dispersed coating
material.
Figure
6 is a copy of Figure 1, the test panel from a finger rub test of a
paint that has a poor dispersion of the pigment particles. In
this paint, some of the pigment particles are present in loosely bound
clusters, they are flocculates.
A
few minutes after the application of the air-drying pigmented coating,
the surface of the coated panel is gently rubbed by a finger, using a
circular movement.
The
poorly dispersed clusters of pigment particles breakdown during the
rubbing, this increases the level of dispersion in the rubbed
areas. The smaller particle size results in an increase in the
absorption coefficient in the rubbed areas and an increase in the
colour depth.
|

Figure 6: Finger rub test of the state of dispersion.
|
|
H52: Pigmented Materials
Light absorption and Particle Size
|
© James H Nobbs
[Colour4Free]
|